Undoubtedly physics is one the most interesting subject but it can be too strict when it comes to concepts. Thermodynamics is a branch of physics which can be a bit tricky to understand. The assignments may demand depth knowledge of concepts and their practical touch. Our thermodynamics assignment help will be the perfect solution to your problems.
Online Assignment Expert is a platform standing with an efficient team of thermodynamics assignment experts to support you academically. We will not only provide help with assignments but will also make sure that you have 100% concept clarity. Our work is plagiarism-free which means we will deliver your unique content. It will help you bad the HD grades and you will be in the good list of your professor. We also provide you with a useful resource for your academic knowledge. Like this one, while you scroll down you can see many key concepts explained to you.
Don't believe anyone who says they are best until they prove so our experts have brought you this sample assignment. This will prove the words that Online Assignment Expert is best for you in every way. The sample will be a useful resource for your assignment help and can also be seen as the source to see our quality work.

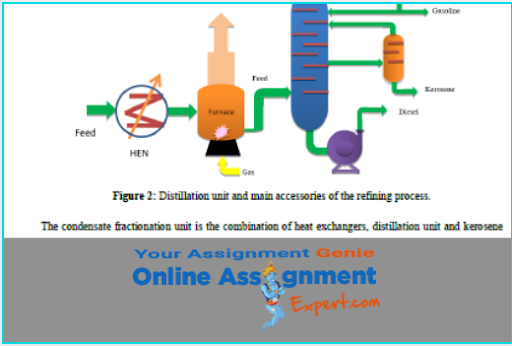
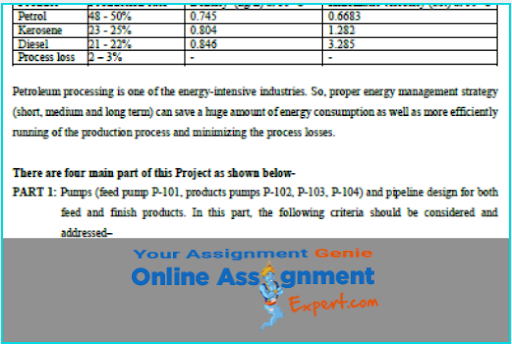
Thermodynamics is a branch of physics which means you might be face problems with other concepts too. You may require other topics assignment help or Physics assignment help. Well don’t think twice and come to us, our experts will help you with every academic problem.
Thermodynamics is not only a branch in physics but it is based on many applications around us that make our life easier. This subject and its principles are the core reasons for the existence of the many resources that we use. Here we will discuss the laws of thermodynamics on which the complete subject is based on. These laws are the origin of many more concepts so this is good for your thermodynamics assignment help online.
Let’s assume there are three systems A, B, and C. Among these systems when system A and B builds a thermal equilibrium individually with the system C. Then at this point both A and B are said to be in thermal equilibrium with each other also. This is the zeroth law of thermodynamics. Its application can be seen with thermometers which help us in measuring our body temperature.
Now the first law of thermodynamics says that: when there is a change in the internal energy of the system then it is equivalent to the heat that is present in the surrounding of that system. And is also equal to the work that is done on the surrounding by the system, it is also known as “law of conservation of energyâ€.
The thermodynamics second law talks about the heat. It states that at a given temperature heat cannot be converted into work completely. It is because the heat does not flow suddenly from colder to hotter regions. As a result entropy with a closed system increases with the time to reach some value which is the maximum. So, an equilibrium state is acquired by every closed system in which the entropy rises to a maximum and no work can be done because of zero energy available.
Now we will quickly scan the third law of thermodynamics which states that: which the temperature tends to zero it means that the entropy of an element within a crystal is stable. This brings out the result that the disorder in the system or any degree of randomness is established by the scale of entropy which is absolute.
History of any subject or topic makes you meet the root of the fruitful tree it is today. This is useful to help with thermodynamics assignment as you will witness the changes this concept has made. You will be getting a glance of the grandness it holds so let us begin:
Thermodynamics is a world within itself it’s essential to cover all aspects and dividing them into different branches would make it easy. The decision is done based on their fundamental properties, their uses, and their theories. These wills enlighten you completely regarding all the cores of thermodynamics. Here we will start the discussion about the types of thermodynamics which are:
Well, you would have already witnessed the sample assignment above that was brought to you by our thermodynamics assignment experts. The format we follow is what your university ask for, along with the marking rubrics. While scrolling this page you would have gone through some important parts of these concepts that you require to know. There is more than this and our assignment maker will help you with all, any kind of academic help related to thermodynamics will be supported.
Not only this topic we cover almost all the subjects and their subtopics and have specified experts for all of them. All you need is to come to us with your problem and we will support you. We strictly follow academic integrity so you won’t find any kind of plagiarism here. We believe in making your life easy so budget is not tension with us. Our prices are student-friendly. Our motive is to make you feel stress –free so you need not worry about your identity leakage. As we promise that your identity will be precious to us and we will keep it safe. You can choose your expert which gives you a reason to trust our system. There is still no full-stop on our policies to support you, we have a list of perks that you can enjoy. Click on that order now button and set yourself free from the academic stress.

Get 24x7 instant assistance whenever you need.

Get affordable prices for your every assignment.

Assure you to deliver the assignment before the deadline

Get Plagiarism and AI content free Assignment

Get direct communication with experts immediately.
Secure Your Assignments
Just $10
Pay the rest on delivery*

It's Time To Find The Right Expert to Prepare Your Assignment!
Do not let assignment submission deadlines stress you out. Explore our professional assignment writing services with competitive rates today!
Secure Your Assignment!